The importance of immunotoxicity testing in drug development cannot be overstated. Immunotoxicity testing plays a pivotal role in the comprehensive evaluation of drugs and chemicals during the crucial stages of development. At BRT, our expertise lies in pre-clinical toxicology testing, with a specialized focus on immunotoxicology. Understanding and assessing the impact of these substances on immune responses is of paramount importance, as it enables us to identify potential risks and safeguard patient safety. By utilizing a range of sophisticated in vivo and in vitro models, we can tailor our studies to effectively evaluate immunotoxicity and provide vital insights that inform the development of safer and more effective therapeutic interventions.
While the data we generate is a piece of the puzzle, there are many additional important steps in the drug development process between discovery and marketing.
The process, as visualized in the below graphic, is comprised of five main stages:
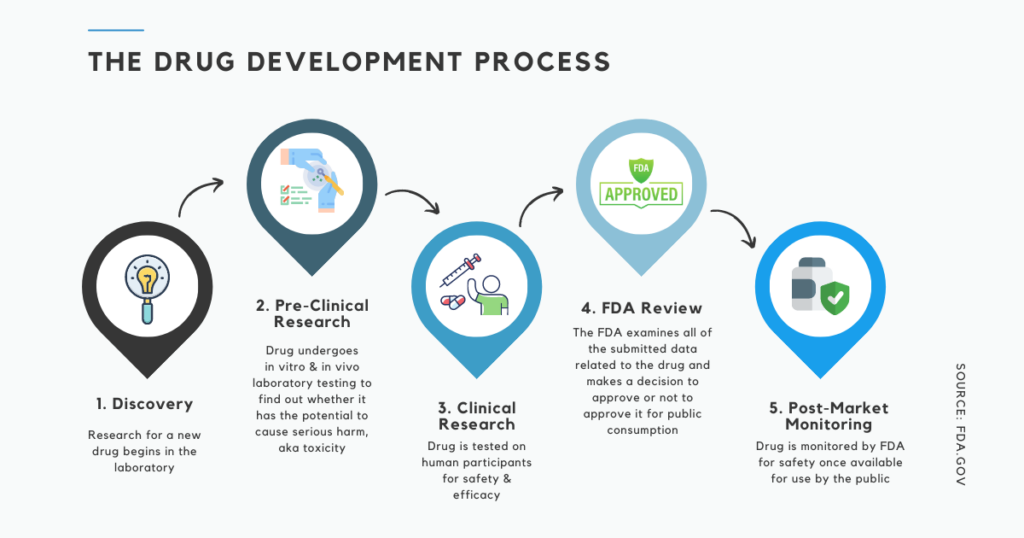
As you can see in the above illustration, in order for a drug to move forward to clinical trials (Step 3), drug manufacturers must provide data to the FDA from pre-clinical pharmacology and toxicology studies that assess whether the product is safe for initial testing in humans.
That’s where BRT comes in.
While pharmacology tests evaluate the short-term safety, bioavailability, distribution, metabolism, and excretion of a drug after administration, toxicology studies aim to discover the effects of long-term drug exposure on the body, including repeat dose studies. These studies look at certain variables like weight, appetite, blood, and urine analysis of a test vehicle pre- and post-dose to evaluate any adverse effects resulting from taking the drug.
At BRT, we specialize in pre-clinical toxicology testing, specifically immunotoxicology. We are primarily interested in the evaluation of immune responses following the administration of a drug, therapy, or chemical. In addition to evaluating safety, our assays also test drug effectiveness. Studies are fully customizable as immunotoxicity can be assessed through multiple in vivo and in vitro models.
The models utilized are carefully selected to detect adverse effects and/or to assess efficacy depending on the agent under investigation. If overall immune function is to be evaluated as a whole, an influenza host resistance or T-dependent antibody response (TDAR) model may be useful, for example. If the agent is a suspected sensitizer, an LLNA, irritancy, or Type I/Type III hypersensitivity model may provide important information. If specific immune cell populations are suspected to be impacted, flow cytometry, natural killer (NK) cell, cytotoxic T lymphocyte (CTL), T cell proliferation, or mast cell/basophil activation assay may be helpful. If cytokine release, complement activation, or the presence of specific biomarkers in a sample may shift following administration or exposure, ELISA or multiplex assays can determine increases or decreases relative to baseline. Many models may be employed to answer questions surrounding immunotoxicity concerns.
In vivo models include:
- Viral (multiple strains, including primary infection and latent viral reactivation)
- Bacterial (multiple strains, including pulmonary, systemic, and wound infection)
- Fungal (multiple strains)
- Co-infection
- T-dependent antibody response (TDAR)
- Antibody dependent enhancement (ADE) of viral replication
- Syngeneic tumor
- Irritancy
- Local lymph node assay (LLNA)
- Type I and Type III hypersensitivity
In vitro models include:
- Functional & screening assays
- ELISA to quantify antibody response to KLH, SRBC, TT, HbsAg from TDAR studies
- SRBC plaque forming cell assay
- Natural killer (NK) cell assay
- Cytotoxic T lymphocyte (CTL) assay
- T cell proliferation assay
- Macrophage/neutrophil function (cytokine production/bactericidal activity)
- Phagocytosis/respiratory burst assay
- Immunophenotyping analysis by flow cytometry
- Whole blood and PBMC immunomodulation assays
- Mast cell/basophil activation assays
- Skin sensitization (DPRA, KeratinoSens, LuSens, hCLAT, GARD) assays
- Cytokine/chemokine/complement/biomarker assays
Although immunotoxicology is our specialty, we offer additional testing in other areas, including:
- Efficacy studies as part of drug development and lead selection
- Metabolism studies
- In vivo studies for collection of PK/TK samples
In conclusion, immunotoxicity testing stands as an essential pillar in the rigorous process of drug development. At BRT, we recognize the significance of immune response assessment and the pivotal role it plays in ensuring the safety and efficacy of pharmaceuticals and chemicals. Through our comprehensive range of in vivo and in vitro models, we offer a customizable approach that addresses diverse immunotoxicity concerns. By leveraging our expertise, researchers and developers can confidently navigate the intricate landscape of drug development, armed with the crucial insights needed to make informed decisions that prioritize patient well-being. With immunotoxicity testing as a cornerstone, we pave the way for a future where breakthrough therapies and interventions can transform lives while upholding the highest standards of safety and effectiveness.

